Publications
2025
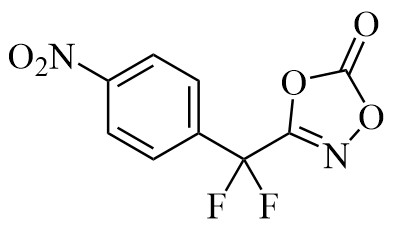
103. 3-[Difluoro(4-nitrophenyl)methyl]-1,4,2-dioxazol-5-one (“K-Diox”)
A. Geraci, K. Antien, O. Baudoin Encyclopedia of Reagents for Organic Synthesis (EROS), 2025
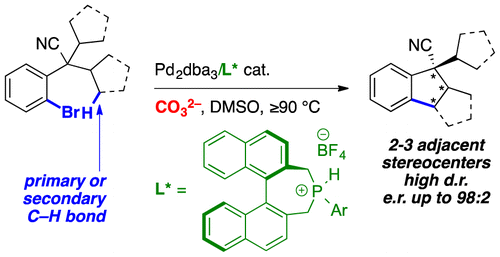
102. A C–H Arylation-Based Enantioselective Synthesis of Planar Chiral Cyclophanes
S. Huh, E. Linne, L. Estaque, G. Pieters, M. Devereux, O. Baudoin. Angew. Chem. Int. Ed. 2025, e202500653

2024
101. Fe-Catalyzed α-C(sp3)–H Amination of N-Heterocycles
A. Geraci, O. Baudoin. Angew. Chem. Int. Ed. 2024, e202417414
Highlighted on Chemistry Views
Highlighted in the Organic Synthesis, Vol. 1, Issue 30

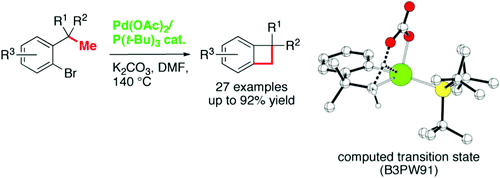
100. 1,4-Pd Migration-Enabled Synthesis of Fused 4‑Membered Rings
M. Tsitopoulou, A. Clemenceau, P. Thesmar, O. Baudoin. J. Am. Chem. Soc. 2024, 146, 18811-18816
Highlighted in OPRD - Org. Process Res. Dev. 2025, 29(3), 603-615
Highlighted in Synfacts 2025, 21, 232
Highlighted by Douglass F. Taber on the Organic Chemistry Portal

99. Total Synthesis of the Diterpenes (+)-Randainin D and (+)-Barekoxide via Photoredox-Catalyzed Deoxygenative Allylation
O. Vyhivskyi, O. Baudoin. J. Am. Chem. Soc. 2024, 146, 11486-11492
Highlighted on the University of Basel News
Highlighted in the Organic Synthesis, Vol. 1, Issue 5
Highlighted in Synfacts 2024, 20, 675.
Highlighted by Douglass F. Taber on the Organic Chemistry Portal

98. Studies towards the Enantioselective Synthesis of Cryptowolinol via Pd0-Catalyzed C(sp3)–H Arylation/Parallel Kinetic Resolution
T. Miyakoshi, D. Kronenberg, S. Tamaki, R. Lombardi, O. Baudoin. Org. Lett. 2024, 26, 2923-2927

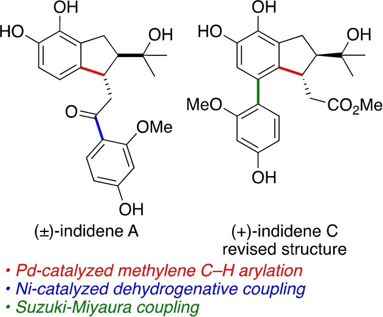
97. Methylene C(sp3)−H Arylation Enables the Stereoselective Synthesis and Structure Revision of Indidene Natural Products
A. Kudashev, S. Vergura, M. Zuccarello, T. Bürgi, O. Baudoin, Angew. Chem. Int. Ed. 2024, 63, e2023161
2023
96. Effect of α-Substitution on the Reactivity of C(sp3)–H Bonds in Pd0-Catalyzed C–H Arylation
M. Wheatley, M. Zuccarello, M. Tsitopoulou, S. A. Macgregor, O. Baudoin, ACS Catal. 2023,13,12563 -12570

95. Iridium(III)-Catalyzed Intermolecular C(sp3)–H Amidation for the Synthesis of Chiral 1,2-Diamines
A. Geraci, U. Stojiljković, K. Antien, N. Salameh, O. Baudoin, Angew. Chem. Int. Ed. 2023, e202309263.

94. A general enantioselective C–H arylation using an immobilized recoverable palladium catalyst
N. Salameh, F. Valentini, O. Baudoin, L. Vaccaro, ChemSusChem 2023, e202300609.
93. Simple synthetic access to [Au(IBiox)Cl] complexes
E. A. Martynova, M. Zuccarello, D. Kronenberg, M. Beliš, A. Czapik, Z. Zhang, K. Van Hecke, M. Kwit, O. Baudoin, L. Cavallo and S. P. Nolan, Dalton Trans. 2023
92. A C–H activation-based enantioselective synthesis of lower carbo[n]helicenes
S. Guo, S. Huh, M. Coehlo, L. Shen, G. Pieters, O. Baudoin, Nat. Chem. 2023
Highlighted on the University of Basel News
Highlighted in Synfacts 2023, 19, 770.

2022
91. Remote Construction of N-Heterocycles via 1,4-Palladium Shift-Mediated Double C–H Activation
T. Miyakoshi, N. E. Niggli, O. Baudoin, Angew. Chem. Int. Ed. 2022, 61, e2021161.
Highlighted in Synfacts 2022, 632.

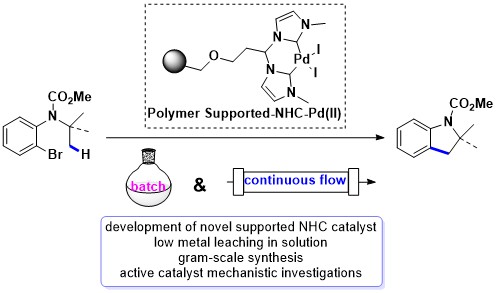
90. C(sp3)–H Arylation Promoted by a Heterogeneous Palladium-N-Heterocyclic Carbene Complex in Batch and Continuous Flow
F. Francesco, I. Anastasiou, N. Salameh, T. Miyakoshi, O. Baudoin, L. Vaccaro, ChemSusChem 2022, 15, e2021027.
2021
89. A Personal Account on Industrial Collaborations in the Field of C–H Activation
O. Baudoin, Chimia 2021, 75, 967–971.
88. Site-Selective Pd-Catalyzed C(sp3)−H Arylation of Heteroaromatic Ketones
A. Kudashev, O. Baudoin, Chem. Eur. J. 2021, 27, 17688-17694.

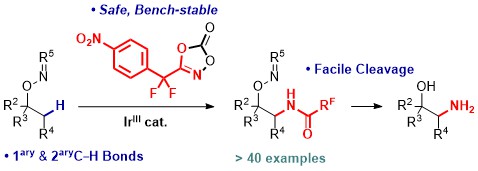
87. A New Dioxazolone for the Synthesis of 1,2-Aminoalcohols via Iridium(III)-Catalyzed C(sp3)–H Amidation
K. Antien, A. Geraci, M. Parmentier, O. Baudoin, Angew. Chem. Int. Ed. 2021, 60, 22948-22955.
Highlighted in Synfacts2022, 17, 1243.
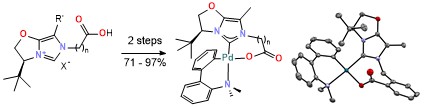
86. Design of Chiral NHC‐Carboxylates as Potential Ligands for Pd‐Catalyzed Enantioselective C–H Activation
N. Niggli, O. Baudoin, Helv. Chim. Acta 2021, doi.org/10.1002/hlca.202100015.
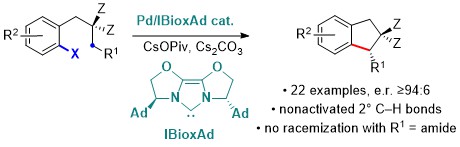
85. Pd0‐catalyzed Enantioselective Intramolecular Arylation of Enantiotopic Secondary C–H Bonds
R. Melot, M. Zuccarello, D. Cavalli, N. Niggli, M. Devereux, T. Bürgi, O. Baudoin, Angew. Chem. Int. Ed. 2021, 60, 7245-7250.
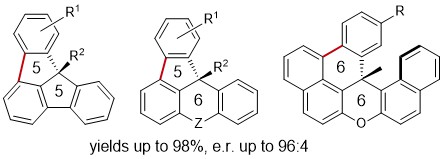
84. Enantioselective Pd0‐Catalyzed C(sp2)–H Arylation for the Synthesis of Chiral Warped Molecules
D. Savary, O. Baudoin, Angew. Chem. Int. Ed. 2021, 60, 5136-5140.
83. Chiral Catalysts for Pd0‐Catalyzed Enantioselective C–H Activation
O. Vyhivskyi, A. Kudashev, T. Miyakoshi, O. Baudoin, Chem. Eur. J. 2021, 27, 1231-1257.
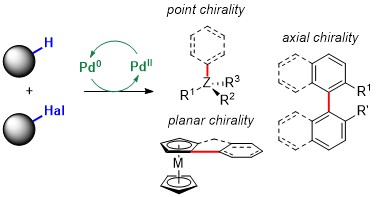
2020
82. Divergent Synthesis of Bioactive Dithiodiketopiperazine Natural Products Based on a Double C(sp3)–H Activation Strategy
P. Thesmar, S. Coomar, A. Prescimone, D. Häussinger, D. Gillingham, O. Baudoin, Chem. Eur. J. 2020, 26, 15298-15312.

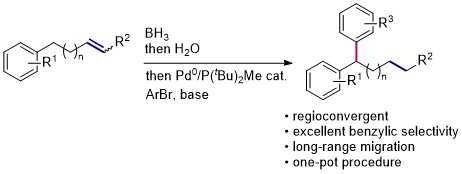
81. One-Pot Alkene Hydroboration/Palladium-Catalyzed Migratory Suzuki-Miyaura Cross-Coupling
Y. Baumgartner, O. Baudoin, ACS Catal. 2020, 10, 10508-10515.
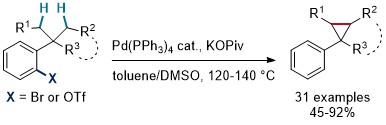
80. Direct Synthesis of Cyclopropanes from gem-Dialkyl Groups through Double C–H Activation
A. Clemenceau, P. Thesmar, M. Gicquel, A. Le Flohic, O. Baudoin, J. Am. Chem. Soc. 2020, 142, 15355-15361.
Highlighted by Jake Yeston in Science.
Highlighted in Synfacts 2020, 1308.
Highlighted on Chem-Station.com.
Highlighted by Douglass F. Taber on the Organic Chemistry Portal.
Abstract on the Organic Chemistry Portal.
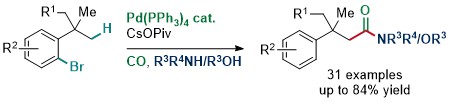
79. Synthesis of Amides and Esters by Pd0-Catalyzed Carbonylative C(sp3)-H Activation
T. Čarný, R. Rocaboy, A. Clemenceau, O. Baudoin, Angew. Chem. Int. Ed. 2020, 59, 18980-18984.
Highlighted in Synfacts 2020, 1193.
78. Multiple Catalytic C–H Bond Functionalization for Natural Product Synthesis
O. Baudoin, Angew. Chem. Int. Ed. 2020, 59, 17798-17809.
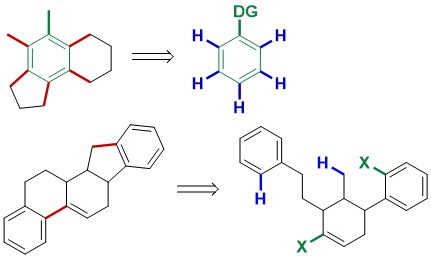
77. Intermolecular Palladium(0)-Catalyzed Atropo-enantioselective C−H Arylation of Heteroarenes
Q.-H. Nguyen, S.-M. Guo, T. Royal, O. Baudoin, N. Cramer, J. Am. Chem. Soc. 2020, 142, 2161-2167

2019
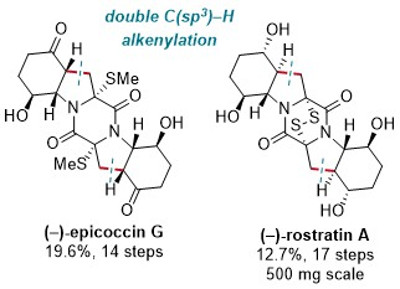
76. Efficient and Divergent Total Synthesis of (−)-Epicoccin G and (−)-Rostratin A Enabled by Double C(sp3)–H Activation
P. Thesmar, O. Baudoin, J. Am. Chem. Soc.2019, 141, 15779-15783.
Highlighted in Synfacts 2019, 1341 and Synfacts 2019, 1423.
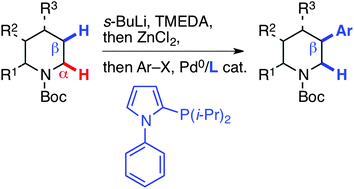
75. Regiodivergent enantioselective C–H functionalization of Boc-1,3-oxazinanes for the synthesis of β2- and β3-amino acids
W. Lin, K.-F. Zhang, O. Baudoin, Nature Catalysis 2019, 2, 882-888
Highlighted by Douglass F. Taber on the Organic Chemistry Portal

74. Redox‐Neutral Coupling between Two C(sp3)–H Bonds Enabled by 1,4‐Palladium Shift for the Synthesis of Fused Heterocycles
R. Rocaboy, I. Anastasiou, O. Baudoin, Angew. Chem. Int. Ed. 2019, 58, 14625-14628.

73. Total Synthesis of (Nor)illudalane Sesquiterpenes Based on a C(sp3)–H Activation Strategy
R. Melot, M. V. Craveiro, O. Baudoin, J. Org. Chem.2019, 84, 12933-12945.
Highlighted by Douglass F. Taber on the Organic Chemistry Portal

72. 1,4-Palladium Shift/C(sp3)-H Activation Strategy for the Remote Construction of Five-Membered Rings
R. Rocaboy, O. Baudoin, Org. Lett. 2019, 21, 1434-1437

71. Divergent Enantioselective Synthesis of (Nor)illudalane Sesquiterpenes via Pd0‑Catalyzed Asymmetric C(sp3)−H Activation
R. Melot, M. V. Craveiro, T. Bürgi, O. Baudoin, Org. Lett. 2019, 21, 812-815

70. Pd-catalyzed γ-arylation of γ,δ-unsaturated O-carbamates via an unusual haptotropic rearrangement
T. Royal, O. Baudoin, Chem. Sci.2019, 10, 2331-2335

2018
69. Domino Pd0‐Catalyzed C(sp3)‐H Arylation/Electrocyclic Reactions via Benzazetidine Intermediates
R. Rocaboy, D. Dailler, F. Zellweger, M. Neuburger, C. Salomé, E. Clot, O. Baudoin, Angew. Chem. Int. Ed. 2018, 57, 12131-12135

68. A Four-Step Synthesis of (±)-γ-Lycorane via Pd0-Catalyzed Double C(sp2)–H/C(sp3)–H Arylation
R. Rocaboy, D. Dailler, O. Baudoin, Org. Lett. 2018, 20, 772-775

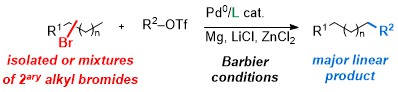
67. Barbier-Negishi Coupling of Secondary Alkyl Bromides with Triflates and
Nonaflates
K.-F. Zhang, F. Christoffel, O. Baudoin, Angew. Chem. Int. Ed., 2018, 57, 1982-1986

66. Chiral Bifunctional Phosphine-Carboxylate Ligands for Palladium(0)-Catalyzed Enantioselective C−H Arylation
L. Yang, M. Neuburger, O. Baudoin, Angew. Chem. Int. Ed., 2018, 57, 1394-1398

2017
65. Synthesis of β-Lactams by Palladium(0)-Catalyzed C(sp3)−H Carbamoylation
D. Dailler, R. Rocaboy, O. Baudoin, Angew. Chem. Int. Ed., 2017, 56, 7218-7222
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
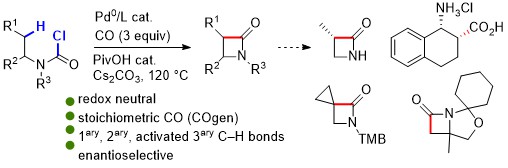
64. Ferrocene derivatives of liquid chiral molecules allow assignment of absolute configuration by X-ray crystallography
P. M. Holstein, J. J. Holstein, E. C. Escudero-Adán, O. Baudoin, A. M. Echavarren, Tetrahedron Asymmetry, 2017, 28, 1321-1329

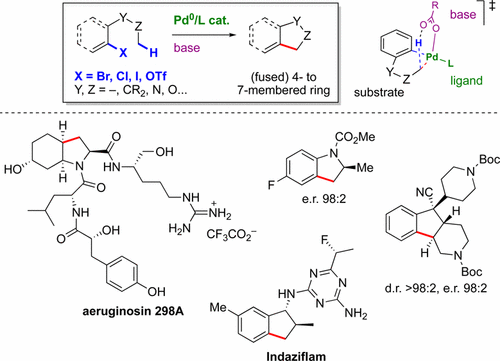
63. Ring Construction by Palladium(0)-Catalyzed C(sp3)−H Activation
O. Baudoin, Acc. Chem. Res. 2017, 50, 1114-1123
62. Palladium(0)-catalyzed asymmetric C(sp3)–H arylation using a chiral Binol-derived phosphate and an achiral ligand
L. Yang, R. Melot, M. Neuburger, O. Baudoin, Chem. Sci.2017, 8, 1344-1349
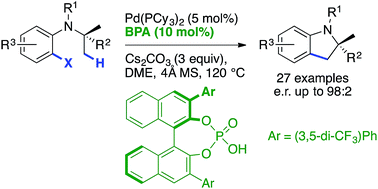
61. Enantioselective α-Arylation of O-Carbamates via Sparteine-Mediated Lithiation and Negishi Cross-Coupling
T. Royal, Y. Baumgartner, O. Baudoin, Org. Lett. 2017, 19, 166-169
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
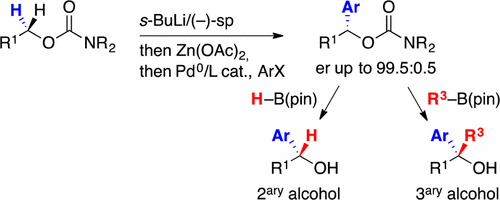
60. Comparative Structural Analysis of Biarylphosphine Ligands in Arylpalladium Bromide and Malonate Complexes
A.-S. Goutierre, H. V. Trinh, P. Larini, R. Jazzar, O. Baudoin, Organometallics, 2017, 36, 129-135
2016
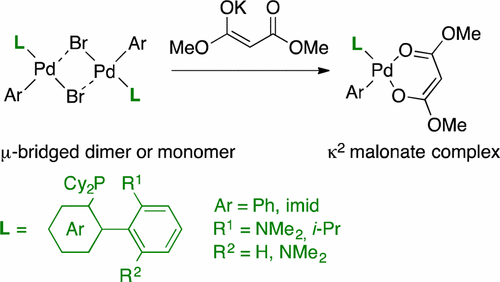
59. Terminal-selective functionalization of alkyl chains by regioconvergent cross-coupling
S. Dupuy, K.-F. Zhang, A.-S. Goutierre, O. Baudoin, Angew. Chem. Int. Ed., 2016, 55, 14793-14797
58. Selectivity Control in the Palladium catalyzed Cross-coupling of Alkyl Nucleophiles
O. Baudoin, CHIMIA2016, 70, 768-772
57. Applications of catalytic organometallic C(sp3)–H Bond functionalization
D. Dailler, G. Danoun, O. Baudoin, in Top. Organomet. Chem. (Eds. : P. H. Dixneuf, H. Doucet), Vol. “C–H bond activation and catalytic functionalization II”, 2016, 133-154
56. Synthesis of Conformationally Constrained Esters and Amines by Pd-Catalyzed α-Arylation of Hindered Substrates
A. Martin, Jean-Pierre Vors, O. Baudoin, ACS Catal. 2016, 6, 3941-3945
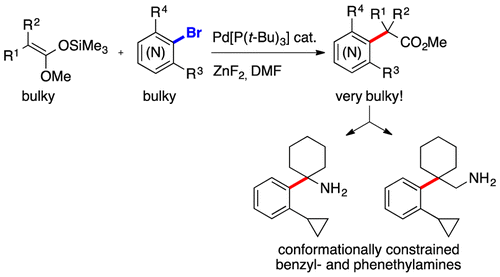
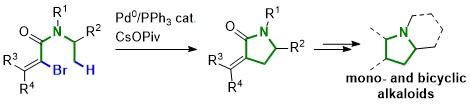
55. Synthesis of Strained γ-Lactams by Palladium(0)-Catalyzed C(sp(3) )-H Alkenylation and Application to Alkaloid Synthesis
P. M. Holstein, D. Dailler, J. Vantourout, J. Shaya, A. Millet, O. Baudoin,* Angew. Chem. Int. Ed.2016, 55, 2805-2809
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
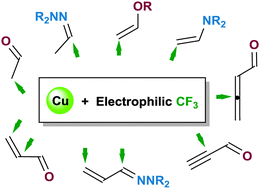
54. Electrophilic trifluoromethylation of carbonyl compounds and their nitrogen analogues under copper catalysis
A.Prieto, O. Baudoin, N.Monteiro, D.Bouyssi, Chem. Commun. 2016, 52, 869-881
2015
53. C–C bond formation by Alkyl C–H activation
O.Baudoin, in Science of Synthesis, catalytic transformations via C–H activation 2 (Ed. : J.Q. Yu), Georg Thieme Verlag KG,2015, Chapter 2.2,37-62
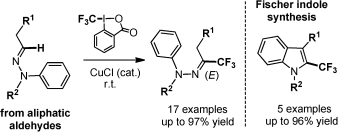
52. Copper-catalyzed trifluoromethylation of aliphatic N-arylhydrazones : A concise synthetic entry to 2-trifluoromethylindoles from simple aldehydes
A.Prieto, M. Landart, O. Baudoin, N.Monteiro, D.Bouyssi, Adv.Synth.Catal. 2015, 357, 2939-2943
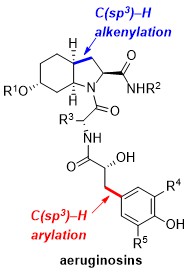
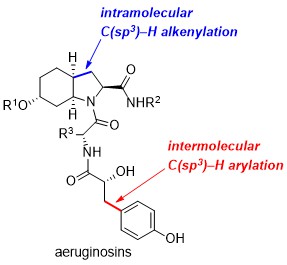
51. Divergent synthesis of aeruginosins based on a C(sp3)–H activation strategy
D. Dailler, G. Danoun, B. Ourri, O. Baudoin, Chem. Eur. J. 2015, 21, 9370-9379.
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
50. Efficient Pd0-catalyzed asymmetric activation of primary and secondary C–H bonds enabled by modular binepine ligands and carbonate bases
P. M. Holstein, M. Vogler, P. Larini, G. Pilet, E. Clot, O. Baudoin, ACS Catal. 2015, 5, 4300-4308
49. Palladium-catalyzed β-selective C(sp3)–H arylation of N-Boc-piperidines
A. Millet, O. Baudoin, Org. Synth. 2015, 92, 76-90
48. A general and scalable synthesis of aeruginosin marine natural products based on two strategic C(sp3)–H activation reactions
D. Dailler, G. Danoun, O. Baudoin, Angew. Chem. Int. Ed.2015, 54, 4919-4922
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
2014
47. Mechanistic study on the selectivity of olefin vs. cyclobutene formation by palladium(0)-catalyzed intramolecular C(sp3)–H activation
C. E. Kefalidis, M. Davi, P. M. Holstein, E. Clot, O. Baudoin, J. Org. Chem.2014, 79, 11903-11910
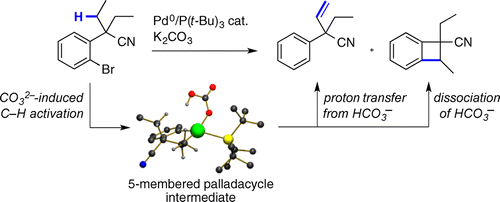
46. Copper-catalyzed β-trifluoromethylation of conjugated hydrazones
A. Prieto, E. Jeamet, N. Monteiro, D. Bouyssi, O. Baudoin, Org. Lett. 2014, 16, 4770-4773
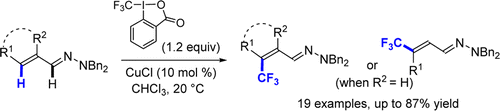
45. Efficient Pd-catalysed allene synthesis from alkynes and aryl bromides via an intramolecular base assisted deprotonation (iBAD) mechanism
N. Nella, E. Parker, J. Hitce, P. Larini, R. Jazzar, O. Baudoin, Chem. Eur. J. 2014, 20, 13272-13278

44. Palladium-catalyzed γ-selective arylation of zincated Boc-allylamines
A. Millet, O. Baudoin, Org. Lett. 2014, 16, 3998-4000
Highlighted in Synfacts 2014, 1190

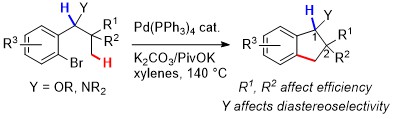
43. Synthesis of 1-indanols and 1-indanamines by intramolecular palladium(0)-catalyzed C(sp3)–H arylation: impact of conformational effects
S. Janody, R. Jazzar, A. Comte, P. M. Holstein, J.-P. Vors, M. J. Ford, O. Baudoin, Chem. Eur. J. 2014, 20, 11084-11090
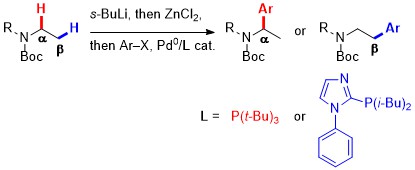
42. Ligand-controlled arylation of acyclic N-Boc-amines
A. Millet, D. Dailler, P. Larini, O. Baudoin, Angew. Chem. Int. Ed. 2014, 53, 2678-2682
Highlighted in Synfacts 2014, 524
41. C-H Bond Alkylation (Including Hydroarylation of Alkenes)
L. Jean-Gérard, R. Jazzar, O. Baudoin, in Metal Catalyzed Cross-Coupling Reactions and More (Eds.: A. De Meijere, S. Bräse, M. Oestreich), Wiley-VCH, Weinheim, 2014, Vol. 3, chapter 19, 1427-1493
2013
40. Palladium-catalyzed β-arylation of silyl ketene acetals and application to the synthesis of benzo-fused δ-lactones
S. Aspin, L. López-Suárez, P. Larini, A.-S.Goutierre, R. Jazzar, O. Baudoin, Org. Lett.2013, 15, 5056-5059
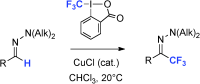
39. Copper-catalyzed trifluoromethylation of N,N-dialkylhydrazones
E. Pair, N. Monteiro, D. Bouyssi, O. Baudoin, Angew. Chem. Int. Ed. 2013, 52, 5346-5349
38. Ligand-controlled β-selective C(sp3)–H arylation of N-Boc-piperidines
A. Millet, P. Larini, E. Clot, O. Baudoin, Chem. Sci. 2013, 4, 2241-2247
37. Intramolecular PdII-catalyzed dehydrogenative C(sp3)–C(sp2) coupling: an alternative to Pd0-catalyzed C(sp3)–H arylation from aryl halides?
C. Pierre, O. Baudoin, Tetrahedron (Symposium-in-Print on Metal-Catalyzed C–H Functionalization)2013, 69, 4473-4478

2012
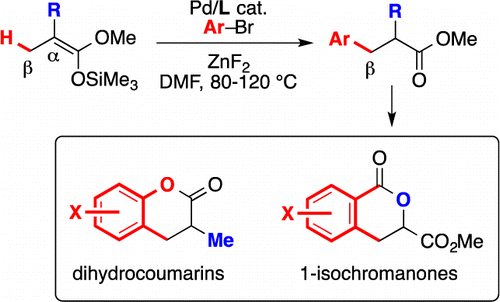
36. Synthesis of aromatic α-amino esters: Pd-catalyzed long-range arylation of primary C(sp3)–H bonds
S. Aspin, A.-S. Goutierre, P. Larini, R. Jazzar, O. Baudoin, Angew. Chem. Int. Ed.2012, 51, 10808-10811
Highlighted in Synfacts 2013, 81
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
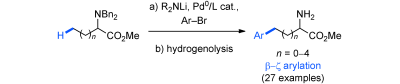
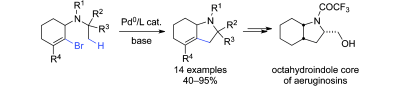
35. Synthesis of hexahydroindoles by intramolecular C(sp3)–H alkenylation - Application to the synthesis of the core of aeruginosins
J. Sofack-Kreutzer, N. Martin, A. Renaudat, R. Jazzar, O. Baudoin, Angew. Chem. Int. Ed.2012, 51, 10399-10402
Highlighted by Douglass F. Taber on the Organic Chemistry Portal
34. Synthesis of cyclobutarenes by palladium-catalyzed C(sp3)–H bond arylation: preparation of methyl 7-methylbicyclo[4.2.0]octa-1,3,5-triene-7-carboxylate
M. Davi, A. Comte, R. Jazzar, O. Baudoin, Org. Synth.2012, 89, 510-518
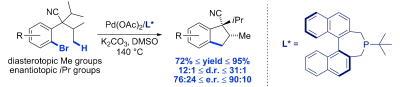
33. Diastereo- and enantioselective intramolecular C(sp3)–H arylation for the synthesis of fused cyclopentanes
N. Martin, C. Pierre, M. Davi, R. Jazzar, O. Baudoin, Chem. Eur. J.2012, 18, 4480-4484
Highlighted in Synfacts 2012, 755
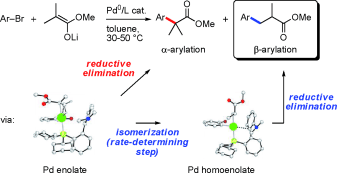
32. On the mechanism of the palladium-catalyzed β-arylation of ester enolates
P. Larini, C. E. Kefalidis, R. Jazzar, A. Renaudat, E. Clot, O. Baudoin Chem. Eur. J.2012, 18, 1932-1944
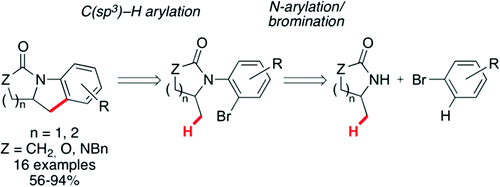
31. Synthesis of tricyclic nitrogen heterocycles by a sequence of palladium-catalyzed N–H and C(sp3)–H arylations
M. Guyonnet, O. Baudoin, Org. Lett.2012, 14, 398-401
2011
30. Transition metal-catalyzed arylation of unactivated C(sp3)–H bonds
O. Baudoin, Chem. Soc. Rev.2011, 40, 4902-4911 (review, special issue on "Cross coupling reactions in organic synthesis")
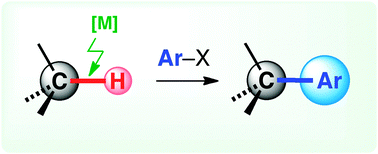
29. Synthesis of polycyclic molecules by double C(sp2)–H/C(sp3)–H arylations with a single palladium catalyst
C. Pierre, O. Baudoin, Org. Lett. 2011, 13, 1816-1819
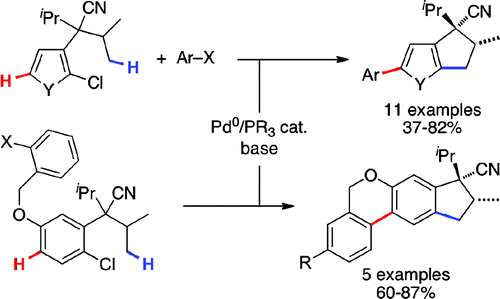
2010
28. DFT study of the mechanism of benzocyclobutene formation by palladium-catalysed C(sp3)–H activation: role of the nature of the base and the phosphine
C. E. Kefalidis, O. Baudoin, E. Clot, Dalton Trans.2010, 39, 10528-10535 (special issue on "Catalytic C-H and C-X bond activation")
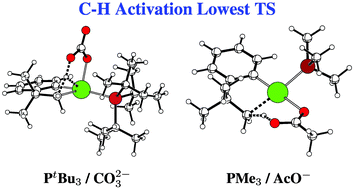
27. The palladium-catalyzed β-arylation of carboxylic esters
A. Renaudat, L. Jean-Gérard, R. Jazzar, C. E. Kefalidis, E. Clot, O. Baudoin, Angew. Chem. Int. Ed.2010, 49, 7261-7265
VIP - Highlighted in Synfacts 2010, 1409

26. Intramolecular palladium-catalyzed alkane C–H arylation from aryl chlorides
S. Rousseaux, M. Davi, J. Sofack-Kreutzer, C. Pierre, C. E. Kefalidis, E. Clot, K. Fagnou, O. Baudoin, J. Am. Chem. Soc.2010, 132, 10706-10716
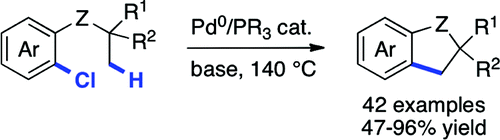
25. Synthesis of antimicrotubule dibenzoxepines
V. Colombel, A. Joncour, S. Thoret, J. Dubois, J. Bignon, J. Wdzieczak-Bakala, O. Baudoin, Tetrahedron Lett.2010, 51, 3127-3129
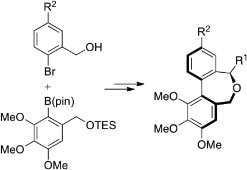
24. Functionalization of organic molecules by transition-metal catalyzed C(sp3)–H activation
R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J.2010, 16, 2654-2672 (review)
2009
23. Synthetic approaches to amino analogues of N-acetylcolchinol
V. Colombel, O. Baudoin, J. Org. Chem. 2009, 74, 4329-4335
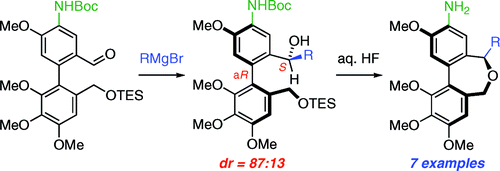
22. Cycloadditions of 1,1-disubstituted benzocyclobutenes obtained by C(sp3)–H activation
M. Chaumontet, P. Retailleau, O. Baudoin, J. Org. Chem.2009, 74, 1774-1776
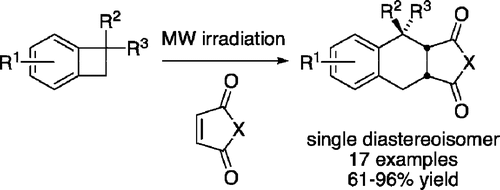
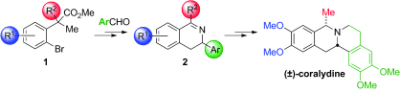
21. Synthesis of 3,4-dihydroisoquinolines by a C(sp3)–H activation/electrocyclization strategy: total synthesis of coralydine
M. Chaumontet, R. Piccardi, O. Baudoin, Angew. Chem. Int. Ed.2009, 48, 179-182
2008
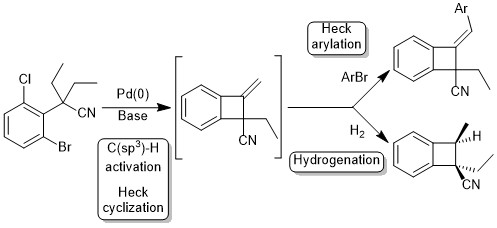
20. Synthesis of benzocyclobutenes by palladium-catalyzed C–H activation of methyl groups: method and mechanistic study
M. Chaumontet, R. Piccardi, N. Audic, J. Hitce, J.-L. Peglion, E. Clot, O. Baudoin, J. Am. Chem. Soc. 2008, 130, 15157-15166.
Highlighted by Douglass F. Taber on the Organic Chemistry Portal.
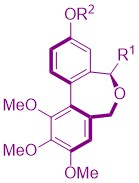
19. Synthesis of anti-microtubule biaryls and preliminary evaluation as vascular-disrupting agents
A. Joncour, J.-M. Liu, A. Décor, S. Thoret, J. Wdzieczak-Bakala, J. Bignon, O. Baudoin, ChemMedChem 2008, 3, 1731-1739
18. Substituted benzocarbocycles by palladium-catalyzed cascade reactions featuring a C(sp3)−H activation step
J. Hitce, O. Baudoin, Adv. Synth. Catal. 2007, 349, 2054-2060
17. Synthesis of tubulin-binding bridged biaryls via intermolecular Suzuki coupling
O. Baudoin, C. R. Chimie 2008, 11, 38-42 (account)
2007
16. Asymmetric synthesis of antimicrotubule biaryl hybrids of allocolchicine and steganacin
A. Joncour, A. Décor, J.-M. Liu, M.-E. Tran Huu Dau, O. Baudoin, Chem. Eur. J. 2007, 13, 5450-5465

15. New approaches for the decarboxylative biaryl coupling
O. Baudoin, Angew. Chem. Int. Ed. 2007, 46, 1373-1375
Angew. Chem. 2007, 119, 1395-1397 (Highlight)
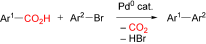
14. A new synthetic approach to biaryls of the rhazinilam type. Application to synthesis of three novel phenylpyridine-carbamate analogues
A.-L. Bonneau, N. Robert, C. Hoarau, O. Baudoin, F. Marsais, Org. Biomol. Chem.2007, 5, 175-183
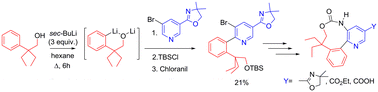
13. Palladium-catalyzed intramolecular C(sp3)–H functionalization: catalyst development and synthetic applications
J. Hitce, P. Retailleau, O. Baudoin, Chem. Eur. J. 2007, 13, 792-799
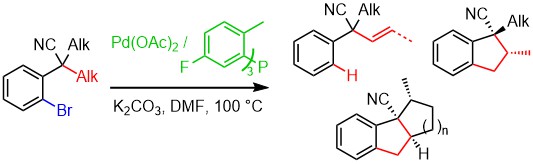
2006
12. Biaryl Axis as a stereochemical relay for the asymmetric synthesis of antimicrotubule agents
A. Joncour, A. Décor, S. Thoret, A. Chiaroni, O. Baudoin, Angew. Chem. Int. Ed. 2006, 45, 4149-4152
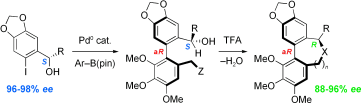
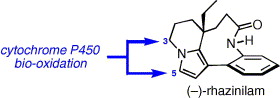
11. Synthesis and biological evaluation of B-ring analogues of (–)-rhazinilam
A. Décor, B. Monse, M.-T. Martin, A. Chiaroni, S. Thoret, D. Guénard, F. Guéritte, O. Baudoin, Bioorg. Med. Chem. 2006, 14, 2314-2332
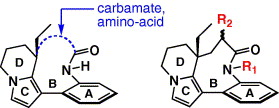
10. In vitro oxidative metabolism study of (–)-rhazinilam
A. Décor, D. Bellocq, O. Thoison, N. Lekieffre, A. Chiaroni, J. Ouazzani, T. Cresteil, F. Guéritte, O. Baudoin, Bioorg. Med. Chem. 2006, 14, 1558-1564
2005
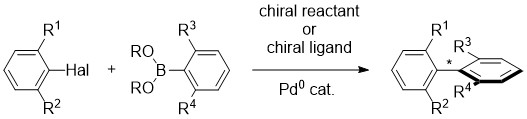
9. The asymmetric Suzuki coupling route to axially chiral biaryls
O. Baudoin, Eur. J. Org. Chem. 2005, 4223-4229 (review)
2004
8. The chemistry and biology of rhazinilam and analogues
O. Baudoin, D. Guénard, F. Guéritte, Mini-Reviews in Organic Chemistry, 2004, 1, 333-341 (review)
2003
7. The palladium-catalyzed C–H activation of benzylic gem-dialkyl groups
O. Baudoin, A. Herrbach, F. Guéritte, Angew. Chem. Int. Ed. 2003, 42, 5736-5740
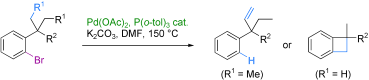
6. A novel 1,3-central-to-axial chirality induction approach to cyclooctadiene lignans
O. Baudoin, A. Décor, M. Cesario, F. Guéritte, Synlett 2003, 2009-2012
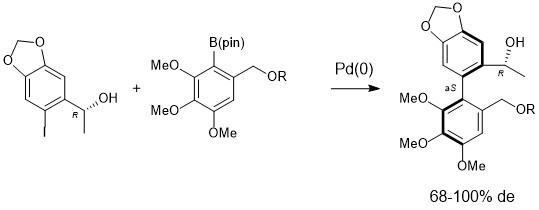
5. Asymmetric synthesis of an axially chiral antimitotic biaryl via an atropo-enantioselective Suzuki cross-coupling
A. Herrbach, A. Marinetti, O. Baudoin, D. Guénard, F. Guéritte, J. Org. Chem. 2003, 68, 4897-4905
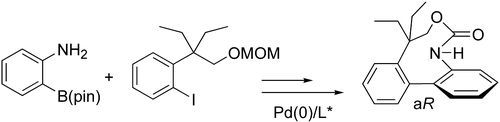
4. Natural bridged biaryls with axial chirality and antimitotic properties
O. Baudoin, F. Guéritte, in Studies in Natural Product Chemistry, vol. 29 (Ed.: Atta-Ur-Rahman), Elsevier, 2003, 355-418
2002
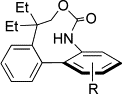
3. Synthesis and biological evaluation of A-ring biaryl-carbamate analogues of rhazinilam
O. Baudoin, F. Claveau, S. Thoret, A. Herrbach, D. Guénard, F. Guéritte, Bioorg. Med. Chem. 2002, 10, 3395-3400
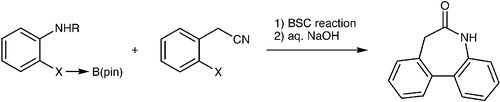
2. Application of the palladium-catalyzed borylation/Suzuki coupling (BSC) reaction to the synthesis of biologically active biaryl-lactams
O. Baudoin, M. Cesario, D. Guénard, F. Guéritte, J. Org. Chem. 2002, 67, 1199-1207
2000
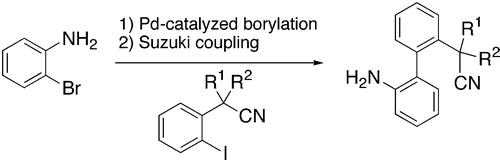
1. Palladium-catalyzed borylation of ortho-substituted phenyl halides and application to the one-pot synthesis of 2,2’-disubstituted biphenyls
O. Baudoin, D. Guénard, F. Guéritte, J. Org. Chem. 2000, 65, 9268-9271